Analysis of Aspirin Tablets Experiment
Determination of Aspirin using Back Titration This experiment is designed to illustrate techniques used in a typical indirect or back titration. Do not lose this container or its contents you will use them later.

Determination Of Aspirin In Tablets Using Back Titration
Objectives In this experiment you will Synthesize a sample of acetylsalicylic acid aspirin.

. This essay sample on Aspirin Titration provides all necessary basic info on this matter including the most common for and against arguments. Use a mortar and pestle to crush enough tablets to produce 1 g tablet powder. Bring samples of two different aspirin brands note names price and the value of the aspirin per tablet indicated by the manufacturer.
To each flask add 20 mL of ethanol. Measure the melting temperature of your aspirin sample. 2-hydroxybenzoic acid salicylic acid being a substituted phenol reacts with Fe3ions to produce a purple colour.
Then 40 mL 10 NaOH is added and refluxed for 15 min. Table 6 Furthermore the price of Bayer. Conduct a colorimetric analysis of your aspirin sample.
Home Resources Lab Experiments Experiment Library College High School Chemistry Analysis of Aspirin Tablets Students use a UV-Vis spectrometer to determine the mass of acetylsalicylic acid ASA in a single aspirin tablet and compare the results to the ASA content stated on the aspirin bottle. Take a small amount of the aspirin tablet and dissolve as much as you can in 3 drops of ethyl acetate. Below are the introduction body and conclusion parts of this essay.
Accurately record the weight of a group of three aspirin tablets so that you can determine an average tablet weight. As seen in Figure 1 Bayer contained 842 Aspirin. Use a mortar and pestle to crush enough tablets to produce 1 g tablet powder.
Errors might be determinate and in-determinate it depends on how large the error is made. The chemical name for aspirin is Acetyl salicylic acid ASA. Using a clean dry weighing bottle weigh accurately by difference triplicate 03 g samples of tablet into labeled 250 mL Erlenmeyer flasks.
Measured yield obtained was 0335g which shows there was an error In the determination. To each flask add 20 mL of ethanol. The aspirin crystals should be firmly packed and fill the capillary tube to a depth of no.
Aspirin Synthesis and Analysis Revised. The determination will be conducted if the acid possesses a robust of feeble aspect in accordance with the Lewis and Brønstead Lowry. Using 05 g of crushed powdered sample N2 NaOH N2 HCl std.
It is used as a fever reducer pain reliever and has antiplatelet effects. Transfer the solution quantitatively into a 250 mL standard flask made upto the mark. He recorded the results in the following data table.
View Lab Report - ANALYSIS OF ASPIRIN TABLETS from CHEM 213 at University of the Fraser Valley. Then add 50 mL of brominating mixture shake well for 15min. Chemistry 12 12Oct2011 Titration- Analysis of Aspirin Tablets Objective.
Other solution concentration units that will be used in this experiment. The analysis shows that they all usually contain the same amount of aspirin usually 300 mg per tablet but the branded tablets contain other ingredients so contain less aspirin in terms of percentage by mass. From that 20 mL is pipette acidified with 2 mL con.
Calculate the percent yield of your synthesis. The results of the experiment found that Bayer had the highest percentage of Aspirin compared to the other tablets. Using a clean dry weighing bottle weigh accurately by difference triplicate 03 g samples of tablet into labeled 250 mL Erlenmeyer flasks.
121314 Crush an aspirin tablet and place in a labeled pre-weighed vial or test tube. The colour is matched against that produced by a set of standard. ANALYSIS OF ASPIRIN TABLETS INTRODUCTION Pharmaceutical.
_ EXPERIMENT 7. Experimental general chemistry 103. As is common with drugs made into pill form the commercial preparations.
It is a drug that was first isolated in Germany by the Bayer Company in 1897. Spectrophotometric analysis of a commercial Aspirin tablet. Tap the closed end of the capillary onto the bench top so that the aspirin crystals work their way to the bottom.
Analysis of Aspirin Tablets 2032 views Jun 17 2021 22 Dislike Share Save pascoscientific 578K subscribers Learn how a UV-Vis Spectrometer can be used. Possible errors could have occurred during the selection of the wavelength 536 nm or during usage of the cuvette where it could have become foggy. B Estimation of aspirin.
Figure 10 Hydrolysis of Aspirin In this experiment the concentration of acetylsalicylic acid in aspirin can be found by running double-beam ultraviolet absorption spectrophotometry. Weight out accurately one tablet into a 50 cm3 conical flask and dissolve it in 100cm3 of 95 alcohol. Tablets 03 g aspirin each recorded the weight of the powder as 65 g he quantitatively analyzed aspirin by back titration.
PubChemDatabaseAspirin CID2244 here back titration was done to obtain the concentration of aspirin from known aspirin tablets amount of product expected was 05g. The class average for the mass of aspirin in the tablet was 365 mg and the average percent of ASA in the tablet was 96. Weight of the powdered aspirin used 05 g _____.
Determine the percentage of aspirin acetylsalicylic. It is an interesting fact that if you buy generic aspirin tablets they are very much cheaper than packets of branded aspirin. Whereas Alka-Seltzer and Countre-Douleurs Plus contained 437 and 626.
The average percent error was 10 which is higher than the percent error of this experiment 3. The aim of this experiment is to ascertain the number of moles of aspirin acetylsalicylic acid which is manifest in five distinct tablets providing titration for the compound with a sodium hydroxide solution 0100 dm-3 as a base. In addition Bayer was the smallest tablet compared to Alka-Seltzer and Countre-Douleurs Plus.
You will be graded on your accuracy. Sensors and Equipment This experiment features the following sensors and equipment. Record the mass of the aspirin tablet.
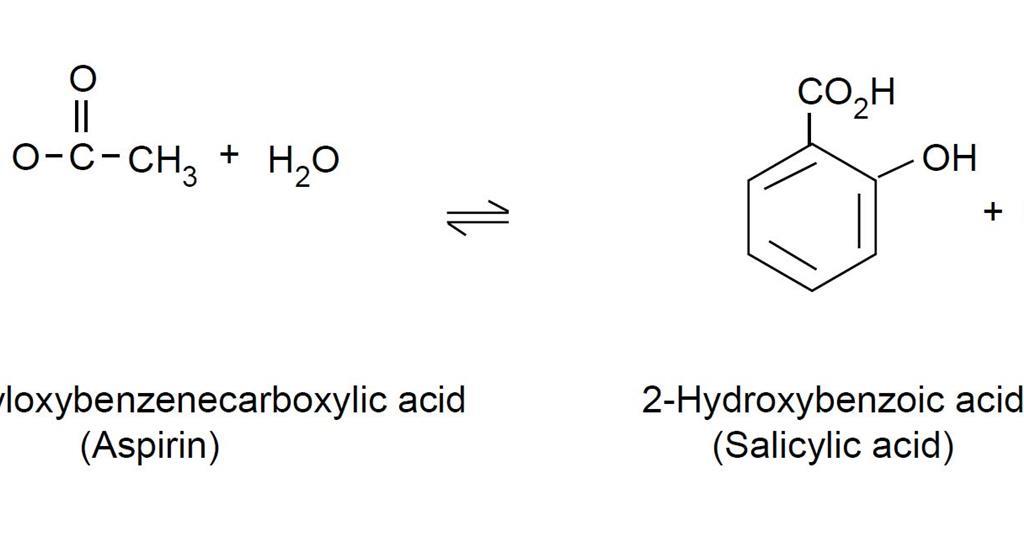
Obtain a capillary tube from your instructor and gently press the open end into the pile of aspirin crystals on the paper so that a few crystals of aspirin enter the capillary tube. Add another 10 drops of ethyl acetate. In this experiment students measure the amount of free 2- hydroxybenzoic acid salicylic acid in 2-ethanoyloxybenzenecarboxylic acid aspirin tablets.
Titrate with 010 mol dm-3 sodium hydroxide solution using two drops of phenolphthalein solution as indicator in the. You will use the NaOH you standardized last week to back titrate an aspirin solution and determine the concentration of aspirin in a typical analgesic tablet. 15g aspirin is weighed out into an RB flask.
Accurately record the weight of a group of three aspirin tablets so that you can determine an average tablet weight.

Experiment Acid Base Titration Of Aspirin

Aspirin Tablets Titration Titration Of Aspirin Tablets In This Lab You Will Determine The Percent Studocu
Analysis Of Aspirin Tablets On A Microscale Experiment Rsc Education

Experiment V02 An Analysis Of Aspirin Tablets
0 Response to "Analysis of Aspirin Tablets Experiment"
Post a Comment